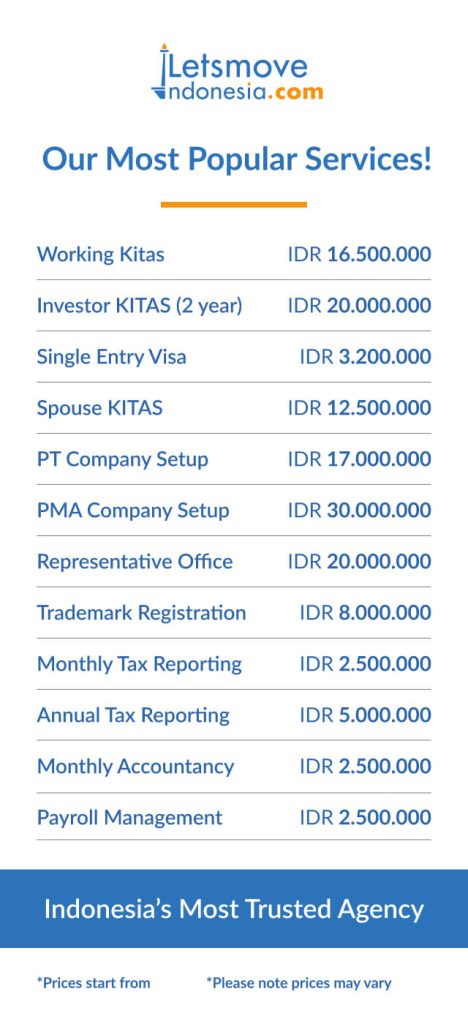
Indonesia’s burgeoning consumer markets, particularly in food, cosmetics, and pharmaceuticals, present lucrative opportunities for foreign businesses. However, navigating the regulatory landscape, especially with the Badan Pengawas Obat dan Makanan (BPOM), is crucial for successful market entry. This guide offers an updated overview of BPOM regulations and the product registration process.
Understanding BPOM: The Indonesian Food and Drug Authority
The BPOM, operating under the Ministry of Health, is responsible for ensuring the safety, quality, and efficacy of food, drugs, cosmetics, and other regulated products circulating in Indonesia. Its mandate includes evaluating products, monitoring compliance, and safeguarding public health.
Regulated Industries and Products
BPOM’s jurisdiction covers a wide range of industries and products, including:
| Category | Description | Examples |
| Cosmetics | Products applied to the human body for cleansing, beautifying, promoting attractiveness, or altering appearance. | Makeup, skincare, hair care, fragrances, deodorants |
| Processed Foods | Food products that have undergone transformation from their natural state, such as cooking, canning, freezing, or other forms of processing. | Snacks, ready-to-eat meals, canned goods, frozen foods |
| Health Supplements | Products intended to supplement the diet and provide nutrients that may be missing or not consumed in sufficient quantities. | Vitamins, minerals, herbs, amino acids |
| Pharmaceuticals | Products intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease. | Prescription drugs, over-the-counter drugs, vaccines |
| Traditional Medicine | Products derived from natural sources used for medicinal purposes based on traditional knowledge and practices. | Herbal remedies, traditional Chinese medicine, Ayurvedic medicine |
BPOM Product Registration Process
Obtaining BPOM certification is mandatory for all regulated products before they can be legally distributed in Indonesia. The process generally involves:
Company Registration
Foreign manufacturers seeking to market their products in Indonesia must establish a legal entity in the country or appoint a local nominee product holder. This process involves submitting a range of documents, including the company’s incorporation certificate, articles of association, and proof of address, to the Ministry of Law and Human Rights.
Product Notification/Registration
Manufacturers must submit detailed product information to the National Agency of Drug and Food Control (BPOM), including ingredients, manufacturing processes, safety data, and labeling. The application must be accompanied by a fee and a sample of the product.
Evaluation and Testing
BPOM conducts risk assessments of the submitted products to ensure their safety and quality. This may involve laboratory testing to evaluate the product’s physical, chemical, and biological properties. The agency may also request additional information or clarification from the manufacturer.
Issuance of Certificate
Upon successful evaluation, BPOM issues a product registration certificate, which is valid for five years and can be renewed. The certificate allows the manufacturer to market the product in Indonesia and provides consumers with assurance of its safety and quality.
Additional Requirements
- Good Manufacturing Practice (GMP) Certification: For certain products, manufacturers must obtain GMP certification to demonstrate adherence to quality control standards.
- Certificate of Free Sale (CFS): For imported products, a CFS from the country of origin is typically required to prove the product’s legal sale in its home market.
Streamlining the Process with Lets Move Indonesia
Navigating BPOM regulations can be complex and time-consuming. Lets Move Indonesia offers end-to-end solutions to streamline your market entry process:
- Regulatory Expertise: Our consultants have in-depth knowledge of BPOM regulations and can guide you through every step of the registration process.
- Company Setup and Nominee Services: We can assist with company registration or provide nominee product holder services to expedite your market entry.
- Product Registration: We handle the preparation and submission of all necessary documentation, ensuring compliance with BPOM requirements.
- Post-Market Support: We offer ongoing support for license renewals, regulatory updates, and compliance monitoring.
Contact Lets Move Indonesia
Partner with us to simplify your BPOM registration and successfully launch your products in the dynamic Indonesian market. Contact us today to discuss your specific needs and embark on your journey to business growth in Indonesia.
Found this article interesting? Check out our other useful articles about visas here!
The Indonesia Visa on Arrival – Everything you need to know now!
Bali Visas – Guide to Bali Visa for UK Citizens
The Indonesia Visa on Arrival 2023 – Everything you need to know now!
LetsMoveIndonesia – 2022 Bali Visas Recap
Jakarta Visas – How to Get a Jakarta Visa on Arrival
Electronic Visa on Arrival (e-VOA) is Now Available For 27 Nationalities